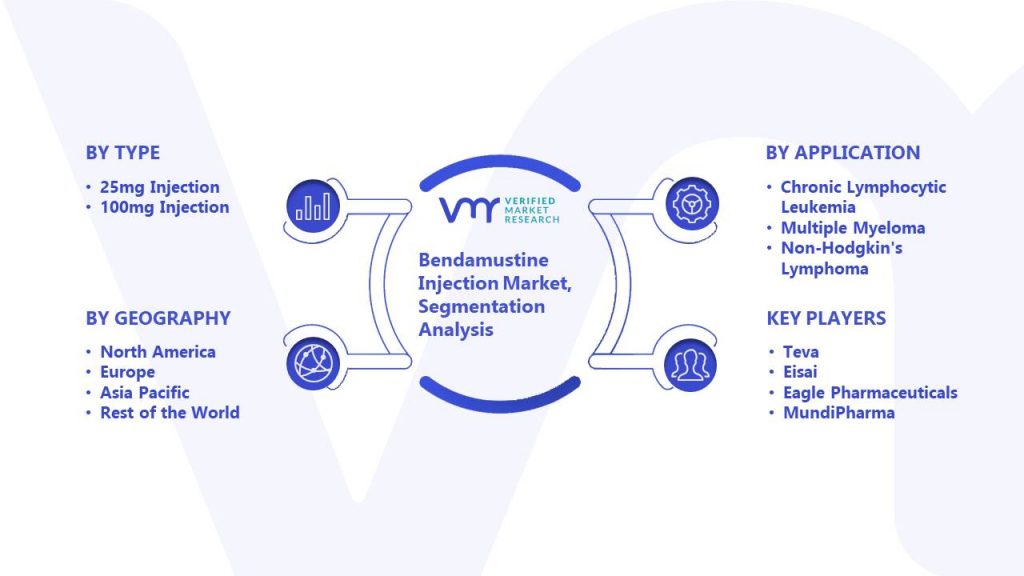
Global Bendamustine Injection Market Size By Type, By Application, By Geographic Scope And Forecast - Verified Market Research

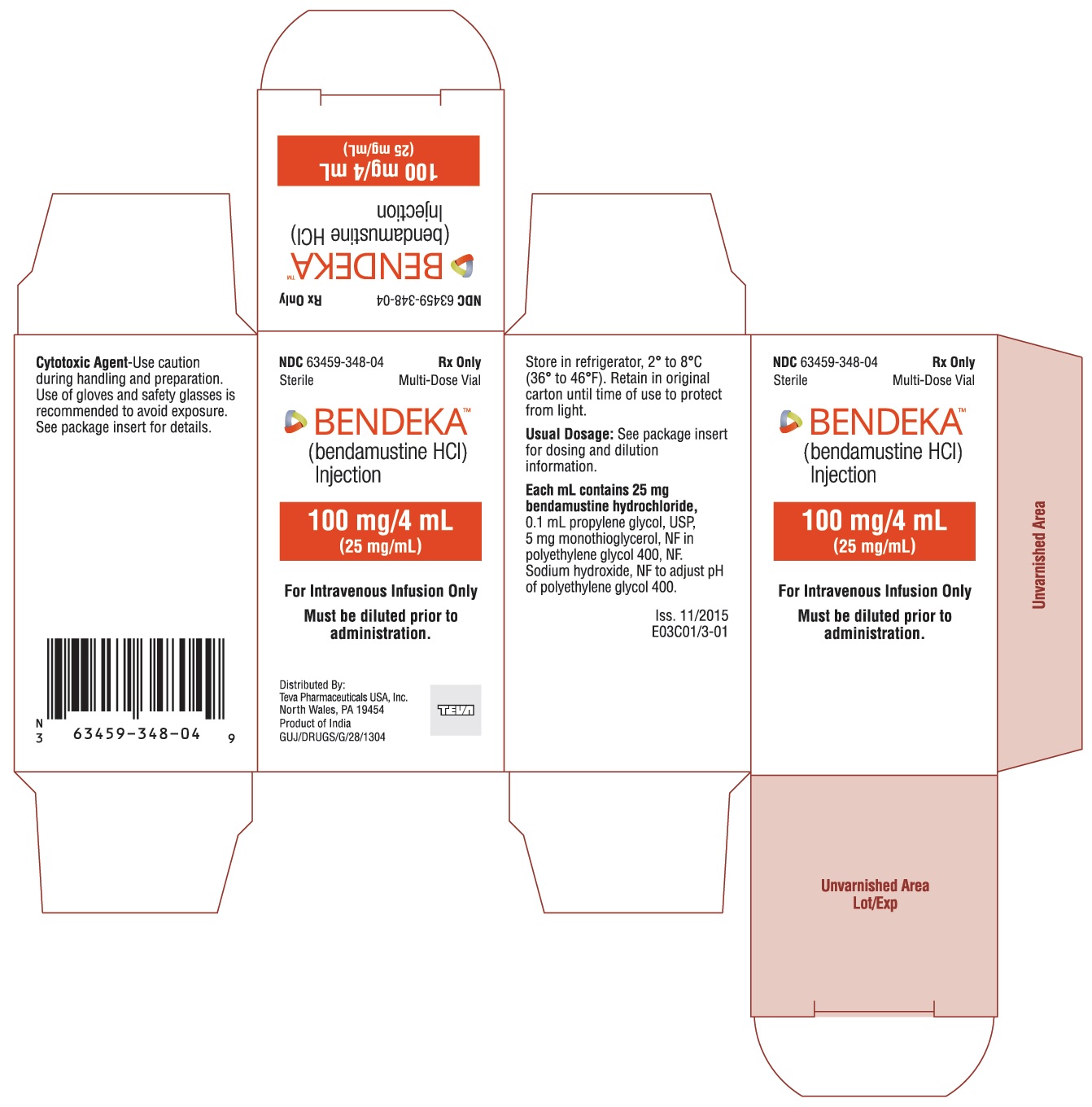
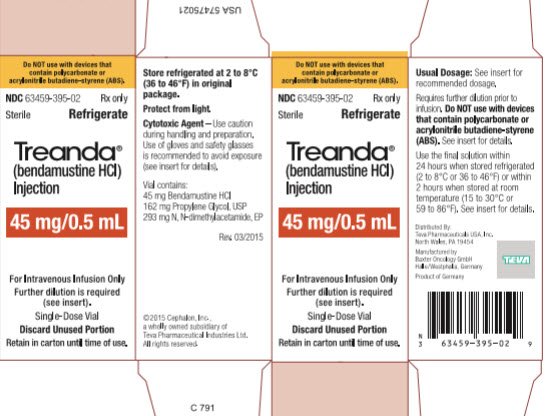
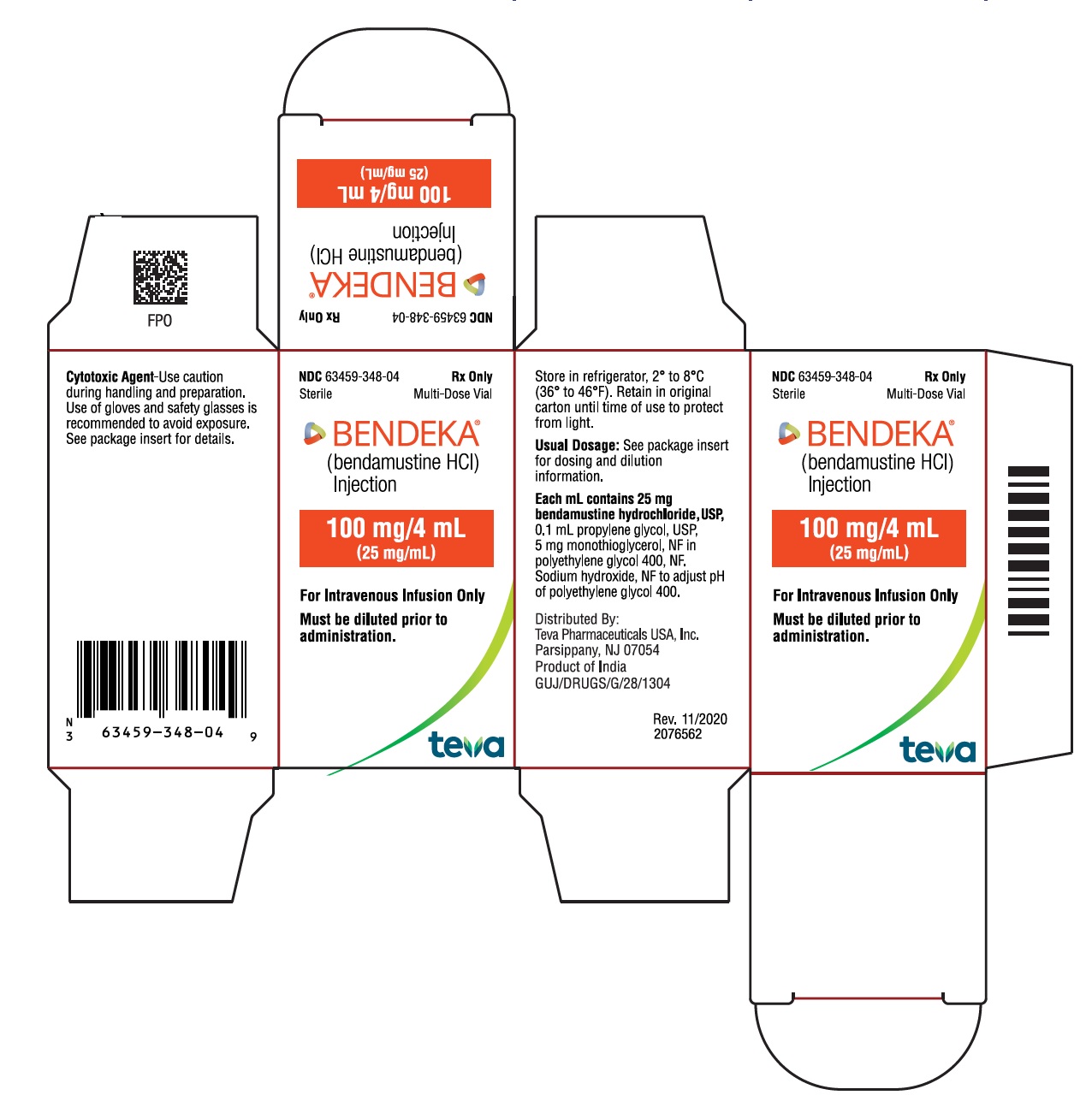
BENDEKA (BENDAMUSTINE HCI) INJECTION Trademark of TEVA PHARMACEUTICALS INTERNATIONAL GMBH - Registration Number 5014704 - Serial Number 86826912 :: Justia Trademarks